Author: Frank Domino, MD in collaboration with Aylin Madore, MD
I stay up-to-date on the latest COVID-19 news and am regularly compiling a list of articles published that are relevant to your primary care practice. Read the insights below from recently published articles in less than two minutes.
Scientific Brief: Community Use of Cloth Masks to Control the Spread of SARS-CoV-2
The CDC updated their recommendations on masks, stating they prevent an infected person from spreading the infection and effectively filter to prevent the mask wearer from contracting an infection.
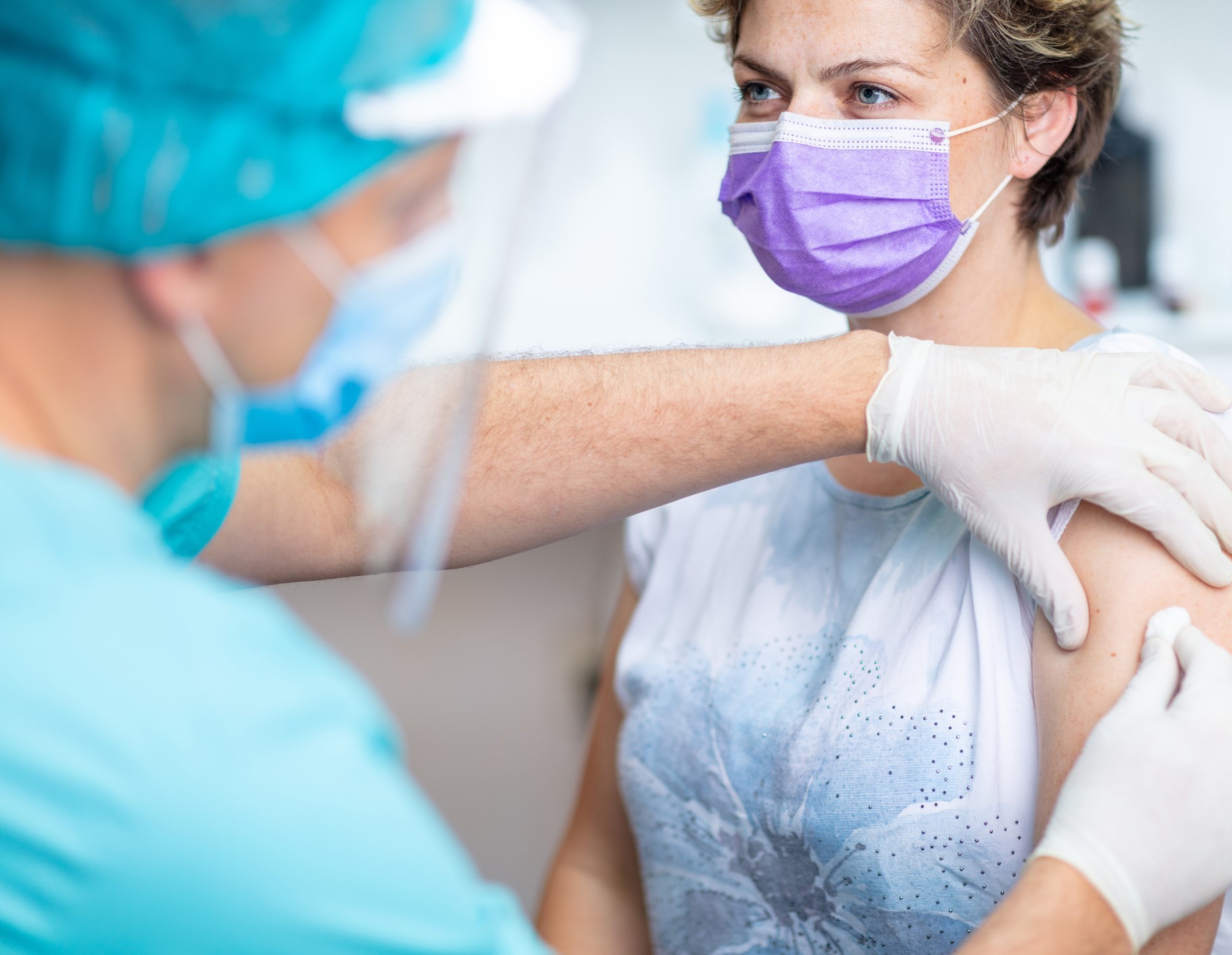
Moderna has Completed Case Accrual for First Planned Interim Analysis of its mRNA Vaccine Against COVID-19 (mRNA-1273)
Moderna, Inc. reports approaching adequate enrollment of their vaccine. Their Phase 3 study included > 7,000 Americans over age of 65 and > 5,000 Americans under 65 with high-risk chronic diseases (diabetes, severe obesity and cardiac disease).
Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis From Phase 3 Study
The SARS-CoV-2 vaccine candidate developed by Pfizer and BioNTech demonstrated an efficacy >90% at 7 days after the second dose is given, based upon a phase 3 trial ~44,000 volunteers.
Coronavirus (COVID-19) Update: FDA Authorized Monoclonal Antibody for Treatment of COVID-19
Bamlanivimab, a monoclonal antibody given emergency authorization by the FDA, is indicated for SARS-CoV-2 positive patients aged 12 years or older who weigh at least 40 kg AND are at increased risk (adults 65 and older and patients with certain medical comorbidities) for progression to severe COVID-19 or hospitalization. A randomized, placebo-controlled, phase 2 trial of bamlanivimab interim analysis at 28 days found the incidence of hospitalization or emergency department visit was ~3% in bamlanivimab recipients vs 10% in the placebo arm. Treatment should be given as soon as possible and within 10 days of symptom onset. It is NOT authorized for hospitalized patients or those who require oxygen therapy.
Safety and Efficacy of Inhaled Nebulised Interferon Beta-1a (SNG001) for Treatment of SARS-CoV-2 Infection: A Randomised, Double-blind, Placebo-controlled, Phase 2 Trial
In a study of 101 patients hospitalized with COVID-19, nebulized interferon beta-1 x 14 days resulted in higher odds of clinical improvement at 15 and 28 days.
For more insights, view our collection of COVID-19 resources and CME courses. We recognize it is critical that you have access to timely, reliable information, so we are working hard to release new content in collaboration with our team of infectious disease experts, medical specialists, and primary care clinicians like Dr. Frank Domino.